The force of attraction between atoms in a molecule, ion, or compound is called intramolecular forces or chemical bonds, these are the very strong force, ionic bonds. A covalent bond and a coordinate covalent bond are the types of intramolecular forces.
Interamolecular Forces
The force of attraction between the molecules of a compound is called intermolecular force, these are weaker attractive forces. it is 25 times weaker than a covalent bond for example an H2O molecule requires 464 KJ/mol energy to break the H - O bonds within a water molecule, while the intramolecular force between a water molecule, requires only 19 KJ/mole energy.
The physical properties of matter depend upon the strength of intermolecular force, the different types of intermolecular force are, dipole-dipole interaction hydrogen bonding London dispersion. These forces are collectively called Vader Wall forces.
{tocify} $title={Table of Contents}
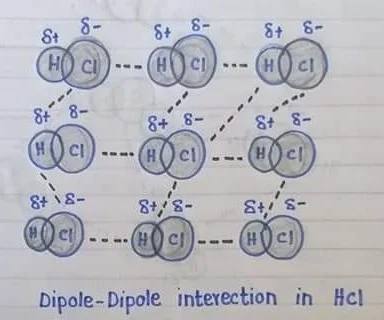
Dipole-Dipole Interaction
The attractive force between the positive pole of one polar molecule and the negative pole of another polar molecule is called dipole-dipole interaction.
Explanation: The forces are found in polar molecules, A polar molecule has two ends or poles due to electro negativities difference of atoms in a polar molecule, one end (pole) of the molecule has a partial positive charge, while the other end has a partial negative charge, positive poles are created at the less electron negative atoms whereas the negative pole is created at the more electronegative atom e.g. H-Cl when polar molecules are close to each other the positive pole of one molecule attract the negative pole of the other molecule, the strength of dipole-dipole forces depends upon the polarity of the molecule, stronger is its dipole-dipole forces and vice versa.
Metallic Bond
The chemical bonding that results from the attraction between metal-positive ions and the surrounding sea of
electrons is called metallic bonding.
Explanation: - The metallic bond cannot be explained based on ions.
Covalent bonds or Vader Walls forces, these are various theories that explain
the metallic bonds the metallic bond can be explained based on Electron
Sea or electron gas theory.
Electron sea Model
According to this model, the valence electrons in metal atoms are loosely held due to the large atoms' size and low ionization energy of metal atoms. Metal atoms lose their valence electrons and become positively charged ions. It means that the outer electron does not belong to only one atom but moves freely between the metal ions like a cloud of negative charge. These mobile electrons form a sea of electrons around the metal atoms which are bled together in metal lattices this sea of electrons bind the metal atoms together forming a metallic bond.
Properties of metallic compound
Atoms of metals are held together by a special type of bond called a metallic bond, metallic compounds show the following
properties.
- All metals are solid at room temperature except mercury which is liquid.
- They have a high melting point and boiling point.
- They are a good conductor of heat and electricity.
- Metals are malleable; they can be beaten into sheets.
- Metals are ductile; they can be drawn into wires.
- Metals are lustrous, they have shiny surfaces.
- Metals are sonorous, they produce a specific ringing sound when struck they have high density.
Properties of Covalent Compound
Those compounds which are formed by
the mutual sharing of valence electrons are called covalent compounds or
molecular compounds. The properties of covalent compounds depend on.
- The geometrical shape of molecules.
- Polarity and inter-molecular forces among molecules.
- Bond type whether single, double, or triple.
Some important properties of
covalent compounds are:
- Covalent compounds have generally low melting points and
boiling points.
- Covalent compounds are nonelectrolytes in their
solution form therefore they do not conduct electricity.
- Covalent compounds are present in all three physical
states solid, liquid, and gas.
- Polar covalent compounds are soluble in polar solvents like water Alcohol etc. while nonpolar covalent compounds are soluble in a non-polar solvent like benzene, acetone, carbon tetrachloride, etc.
- The reaction of covalent compounds is slower than ionic
compounds.
- The bond in a covalent compound is a directional solid
covalent compound that can form a molecular crystal.
Properties Of Ionic Compounds
Ionic compounds are formed strong
electron static force of attraction between the two oppositely charged ions some
important properties of ionic compounds are given below.
- Ionic compounds have high melting and boiling points. due
to the strong force of attraction between ions.
- Ionic compounds are solid at room temperature.
- Ionic compounds are soluble in polar solvents like
water.
- The reaction of ionic compounds is very fast in molten
state or in solution form.
- The bonds in ionic compounds are non-directional.
- Ionic compounds are composed of cations and anions in
crystalline form.
Liquid, Solid, and Intermolecular force
The three states of matter
1. Gases,
most compressible, least dense.
2. Solids, least compressible,
most dense.
3. Liquids In this section well
force on the condensed states.
>Valence bond theory and Molecular orbital theory
>Discovery of Electrons, Protons, Neutrons and the structure of the atom