John Dalton proposed that the atom is the smallest indivisible particle of matter. It contains three subatomic particles called Electrons, Protons, and Neutrons. discovery of particles and atom theory.
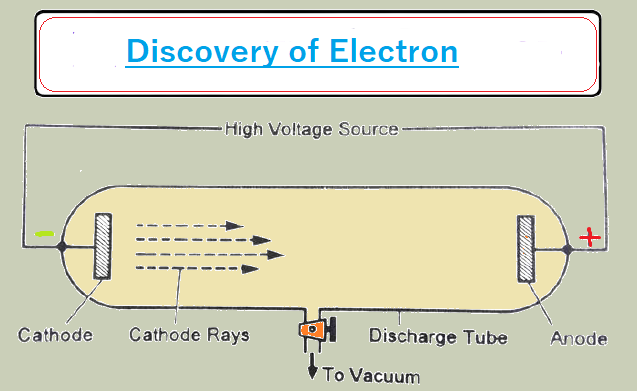
(I) Discovery of electron (Cathode Rays Experiment)
Select the glass tube and vacuum pump it creates ultra-low pressure in the glass tube - 10¯6 mm/Hg and high voltage- 10000-volt cathode and anode. In this condition, rays come out from the cathode to the anode which is called a cathode ray. Cathode rays move -Ve charge and an electric field is applied to cathode rays deflected in the direction of +Ve charge.
The spin wheel is kept in the direction of cathode rays: the wheel rotates. It confirms that cathode rays have
particle no here. create a sharp shadow of an object kept in the direction of cathode
rays. It confirms that cathode rays move in a straight line. initially, these were
k/a mega tons later changed to the electron.
Nature of cathode rays.
The electrons travel from the battery to the cathode and these electrons move from the cathode to the anode in the form of
cathode rays. The nature of the cathode ray means the charge/mass ratio of cathode rays. The nature of cathode rays does not depend on gas filled in a glass tube. and cathode
rays do not depend on the electrode used.
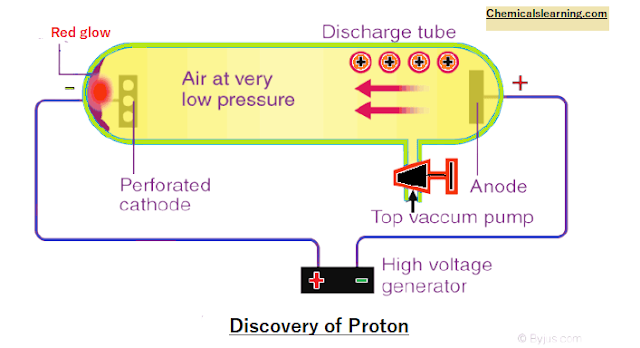
(II) Discovery of Proton (anode ray experiments)
Perforated cathode,
small holes in cathode red glow behind cathode it seems rays are coming from cathode to cathode. cathode rays originate from the cathode but anode rays do not
originate from the anode when voltage is applied e¯ are emitted from the cathode and
move towards the anode.
The electron ionizes gas
filled and +Ve charge ions are formed. +ve ion moves from anode to cathode and is observed as anode rays. therefore, anode rays are not generated from the anode.
Properties of anode rays
For different gases used the charge-to-mass ratio is different. The nature of anode rays (charge / mass ratio)
depends. anode rays travel in a straight line like cathode rays. These also
rotate pinwheel-like cathode rays. Deflected in an electric field: Anode rays are
+Ve charge.
(III) Discovery of Neutron
Discovered by Chadwick,
it is a neutral particle, it is produced by the bombardment of α-particle on
Be Beryllium (Be) atom.
4Be9 + 2He4: 6C12
+ 0η1
Mass of Elementary particles (Important Data)
Mass of electron = 9.1🗙10¯^31 kg and charge of electron = 4.6🗙10¯^19 ( - )
Mass of proton = 1.66🗙10¯^27 Kg and charge of proton = 1.6🗙10¯^19 (+)
Mass of neutron = 1.675🗙10¯^27 kg and charge of neutron = Zero
Rutherford Atomic Model (α-particle scattering experiment)
Rutherford used thin
gold foil for this experiment because gold foil is the most malleable. He
bombarded α-particle on thin gold foil. He made a circular fluorescent screen around the gold foil. Most of the α-particles passed undefeated through the gold
foil. Most of the part of atoms are empty. Few α-particles have deviated through small angles.
Atoms containing -Ve and +Ve
charge in equal amounts atoms are neutral. Very few α-particles deviated back
to the original path (180০ deviated). The whole of the +Ve charge of an atom
is concentrated in a small volume called the nucleus.
Characteristics of Rutherford's model
The size nucleus is very
small as a compound to the size of an atom. R atom = 10¯10 times bigger than a nucleus
R = R。(A) 1/3 cm = R。= 1.33✖10¯^15 m
where
A = Mass number R =
Radius of the nucleus.
The e¯ revives around the nucleus in a different concentric circus, culled orbits. Most of the atoms are
empty.
The drawback of the Rutherford model
Rutherford's sail electron revolves around the nucleus in orbits. The electron is continuously changing its direction, which means the electron is in accelerated motion. A/c to Maxwell if a charged particle rotates around another charged particle in accelerated motion, then if releases energy then finally it will merge in the nucleus, so Rutherford failed to explain the existence of the atom. Rutherford failed to explain Maxwell's electron theory. It cannot explain the line spectrum of hydrogen.
Thomson atomic model
Atoms are considered positively charged spheres, and electrons are distributed uniformly in these spheres. This model is also known plum pudding model. It explains the neutrality of atoms, but can not explain the stability of atoms.
>Types of Chemical Equilibrium and Law of mass action
>Valence bond theory and Molecular orbital theory
>Intramolecular and Intermolecular forces | Metallic Bond