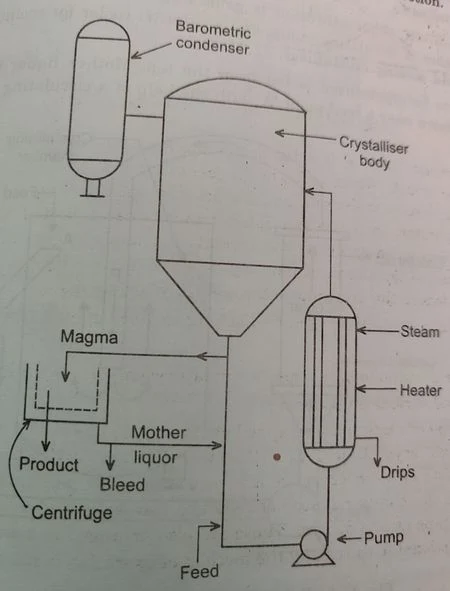
In this vacuum crystallizer supersaturation is achieved by adiabatic evaporative cooling. A hot solution (feed) is introduced into a vessel wherein a vacuum is maintained that corresponds to the boiling point of the solution lower than the temperature of the feed solution.
The energy needed for vaporization is taken from the feed so that the temperature of a liquor-vapor mixture after flashing becomes much lower than the temperature of the liquor before flashing. Vacuum crystallizers are often operated continuously but they can also be operated batch-wise. These crystallizers are very simple and contain no moving parts so they can be constructed out of corrosion resident materials or lead or rubber-lined mild steel.
Construction
A continuous vacuum crystallizer consists of a tall vertical cylindrical vessel with a conical bottom a circulating pump of low head and a vertical tubular heater on the shell side of which steam is condensing. Low pressure in the vertical cylindrical vessel is maintained by a condenser usually with the help of a steam jet ejector.
A tangential inlet is provided on the cylindrical vessel for introducing a hot solution into it and a vapour outlet is provided on the top. A discharge connection for mother liquor and crystal is provided on a downpipe just above the feed connection.
Working Principle
The magma from the bottom of a cylindrical vessel goes to a pump via a downpipe and is pumped through a vertical tubular heater where it is heated using condensing steam and finally, a hot stream enters the cylindrical vessel tangentially just below the level of the magma surface. Flash evaporation of the solution takes place and produces rapid cooling resulting in supersaturation, which is the driving force for nucleation and growth.
The fresh solution enters the downpipe just before the suction of the circulating pump and a suspension of crystals is continuously taken out from a discharge pipe located above the feed inlet in the downpipe. The suspension of crystals is fed to a centrifuge machine, the crystals are taken out as a product and the mother liquor is recycled to the down-pipe with small parts of it continuously bled. It is used for the production of large crystals.
Vacuum Crystallizer Advantages and Disadvantages
Advantages
Vacuum crystallization enables perfect control of the crystallization process, producing crystals of consistently excellent quality.
1. When operating under reduced pressure, the solvent's boiling point is lowered, protecting components that are sensitive to heat.
2. When compared to conventional crystallization techniques, reduced pressure might result in less energy being used.
3. It helps in the purification of products by efficiently separating solutes from solutions.
4. Vacuum operation reduces scale accumulation on equipment, which lowers maintenance requirements.
Disadvantages
1. Due to their unique design, vacuum crystallizers can be costly to buy and maintain.
2. They are less user-friendly than other systems since they require trained operators to set up and maintain them.
3. The applicability of vacuum crystallization is constrained because not all solvents are appropriate.
4. Compared to some other crystallization techniques, the procedure could be slower.
5. Working in a vacuum environment has safety implications, such as the possibility of implosions if not carefully controlled.
The decision to employ a vacuum crystallizer is influenced by the particular requirements of the procedure, the materials involved, and the resources for equipment and experienced labor that are accessible.
Take these Notes is, Orginal Sources: Unit Operations-II, KA Gavhane