Refrigeration means keeping the sequence constant at a lower temperature than the environment. That is, in the refrigeration process, continuous heat is absorbed from the low level and given to the high level. Crude is used in the chemical process industries of refrigeration as liquids process heat removal process etc.
This process is commonly used in the manufacture of synthetic rubber, textile chlorine, plastics, hydrogen fluid, etc.
{tocify} $title={Table of Contents}
Methods of Low-Temperature Achieved
At temperatures lower than that of the atmosphere, the temperature gradient is kept constant by various means.
1. By phase change to remove heat.
2. By expanding the compressed gas.
3. By absorption of gases.
4. Diamagnetism of solids.
5. Dimetal function by the electric current method.
Coefficient of Performance Refrigeration (COP)
The figure shows a refrigeration process, with Q2 heat being absorbed at temperature T2 and Q1 heat being rejected at temperature T1.
Form law of Thermodynamics
External work w = Q1 - Q2
R = Q2/w
R = Q2/Q1 - Q2
The refrigeration capacity determines the power rate of the refrigerator. It is measured in tons of refrigeration and shows heat absorption in tons.
That is, the COP of the Carnot refrigeration cycle depends only on force and not on working fluid, in fact, it is not possible to get any refrigeration machine working on the Carnot cycle. Because the Carnot cycle works on the reversible operation and becomes irreversible in every practical process, its efficiency is high. Q2 is the minimum work required for heat transfer.
W = Q2 (T1 - T2)/T2
Refrigeration Cycle
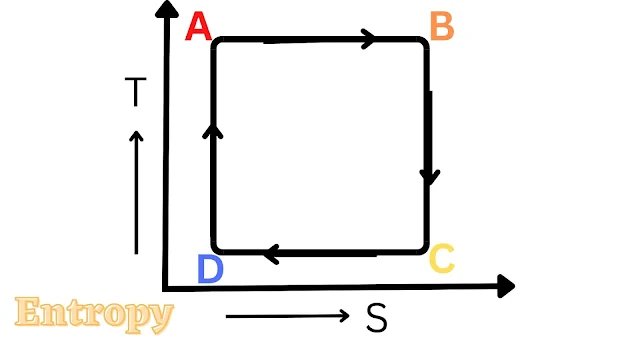
It is a commercially used refrigeration cycle equipment. The air refrigeration cycle is shown in the figure. Air compresses isotropically from A to B and expands from C to D at the material entropy of the air. The work generated in process CD is used in process AB.
The T-S diagram per air cycle is shown. This is an expensive method in which a powerful high compressor is used.
The heat is absorbed by the vaporization of the liquid, and after the operation of the evaporated liquid is completed, it is brought back to its original state. Then it is the main 3 stages of the refrigeration cycle of low-level heating.
1. Absorption of heat at low temperature
2. Removal of heat at high temperature
3. Expansion
Characteristics of an Ideal Refrigeration
The following characteristics are necessary while selecting refrigeration for a particular refrigeration operation.
1. Due to low vapor pressure in the condenser, the cost of dining and maintenance should be less.
2. The vapor pressure of the evaporator should be slightly higher than the atmospheric pressure so that air does not leak into the system as air has a great effect on transfer.
3. It is used to freeze due to the moisture present in the air.
4. The compressor increases the amount of work supplied for the refrigeration work to a certain extent.
5. High latent heat of vaporization and low heat capacity (mass flow rate) reduce it.
6. The energy of saturated vapor remains constant.
7. It should not be of explosive nature.
8. It Should not be soluble in lubrication oil.
9. The non-position of refrigeration of power special is a unique quality.
10. Must be of low cost.
Refrigeration Coefficient
If the Carnot cycle is run in the upward direction, then work has to be done, and keeping the temperature low, heat will come out and heat transfer will happen to the higher temperature source. It is used in refrigerators.
Refrigeration Coefficient = heat removed at lower temperature/work done
E = Q2/Q1 - Q2
Types of Refrigeration Cycle
This is an ideal refrigeration cycle because all refrigeration works according to this. This can be expressed in the following four steps.
1. Reversible adiabatic compression (AB)
2. Isothermal heat reflection (BC)
3. Reversible adiabatic reflection (CD)
4. Isothermal heat Absorption (DA)
Isothermal Heat Absorption
During DA the low-temperature T2 to Q2 heat is absorbed by the area A dry which at T2 is equal to Xs where DS is the change in entropy during heat absorption. Q1 = (T1 ∆s) heat is given to high-temperature T1 during an isothermal heat reaction. area (ABXX) thus heat transfer is external work. It has been shown from the T-S diagram on ABCD.
Thus for the Carnot cycle
COP= Q2/w = Q2/Q1 -Q2
or COP = T1/T1 - T1
Let m = rate of flow of air
COP of air refrigeration cycle Cp = Sp
heat Heat absorbed in refrigeration Q2 = mCp (TA - TD)
The heat removed from COP Q1 = mCp (TB - TC)
w = Q1 - Q2
w = mCp [(TB - TC) - (TA - TD)]
C.P.O = Q2/w
C.P.O = mCp(TA - TD)/mCp [(TB - TC) - (TA - TD)]
C.P.O = TA - TD/(TB - TC) - (TA -TD)
For CD and AB adiabatic process
COP = TA/TB - TA
Working of Vapour Compression Refrigeration Cycles
During this cycle, the liquid in the refrigeration unit evaporates to absorb heat at a certain temperature. The liquid valve is pressed at high pressure, and this gas is fed to the condenser at high pressure, where the heat of the gas is rejected during condensation, now the curve is completed by making the liquid gas flow through the expansion stage carried out, the liquid is brought to its original state. Compression and expression are made more reversible by reducing reversibility.
The expansion engine shown in the image is resampled with a Carnot cycle of small cooling difference from supersaturated steam to supersaturated steam and this is determined before the condensation step.