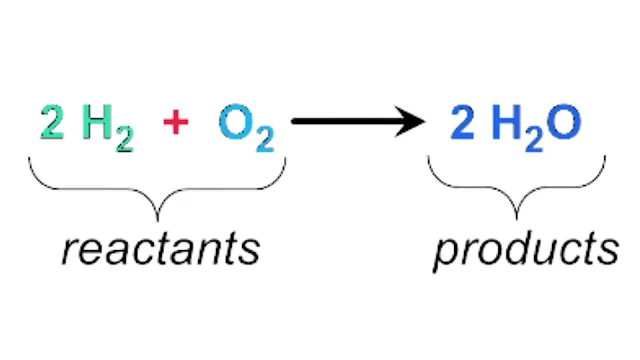
A substance that takes part in a chemical reaction and changes when mixed with another substance. This post ost the types of Reactants and Reactants definition, like Limiting reactant, Excess reactant, Percent of excess reactant, degree of the composition of reactant, and reactant ratio, etc.
Types of Reactants
Limiting Reactant
The reactant present in the chemical process in such proportion that its use limits the extent of the reaction is called limiting reactant.
OR
The reaction whose stoichiometric amount is less than that of other reaction factors is called a limiting reactant.
Excess Reactant
The reactant that is present more than the limiting reactant according to the stoichiometric ratio is called excess reactant.
Percent of Excess Reactant
Percent of excess reactant is calculated from the amount of any reactant which according to the chemical equation is more than the amount required for reaction with the limiting reactant.
Percent Excess Formula
Percent Excess = (moles in excess)/(moles required to react with limiting reactant) x 100
Degree of Completion of Reaction
The partial or percentage amount of limiting reaction that is changed into a product is called the degree of Completion of reaction.
Percent Conversion Chemistry
The change in the percentage amount of any reaction in the product is called percentage conversion.
Reactant Ratio Definition
The number of moles of reactant added to 1 mole of limiting reaction is called the reactant ratio.
Reactant Ratio = mole of excess reactant reacts with Limiting reactant/moles of limiting reactant
Tie Element and Tie Substance
Tie Element
A tie element is a substance that flows from one currency to another without any kind of change. For the selection of the tie element, we see which element remains unchanged in the process with constant mass or weight, that element is the tie element. Sometimes many elements actually flow through the process.
Thus in such a situation, there are many possibilities for the selection of a tie element. Sometimes we do not get tie elements during direct tests, in such situations, there is a need to develop a contract or man-made tie element which is as effective as a single material tie element.
Tie Substance
The substance that appears but has one incoming steam and one outgoing steam as reference for calculation is called a tie substance.
It is an element, compound, or active mass that serves as a reference for calculations. It is also called a key component, ash is a tie element in a coal furnace.
Also Read: Atomic fraction, Volume Fraction, and Pressure Fraction Definition and Formula