Naphtha is a mixture of hydrocarbons, whose boiling point is that of gasoline and kerosene. When we do light distillation of petroleum, as a result of the refinery, we get crude oil with a boiling range of 94 to 232°C which is called naphtha. It is used as feedstock for ammonia production.
{tocify} $title={Table of Contents}
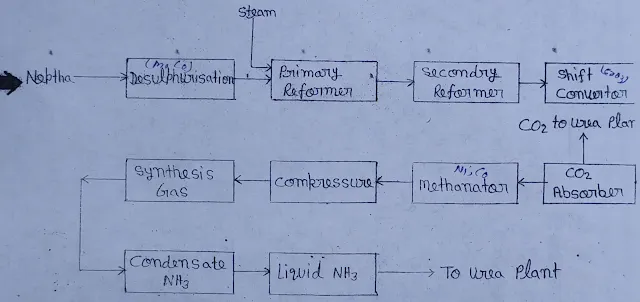
Production Process
In this process, first of all, naphtha is mixed with hydrogen and preheated. Then it is sent to the reactor. In this reactor (Co + Mo) is used as a catalyst. Here sulphur gets hydrogenated and converted into H2S as per the following reaction.
R.S.H + H ⟶ R.H + H2S
R.S.R' + H ⟶ R.H + R'.H + H2S
R.S.S.R' + H ⟶ R.H + R'.H + 2H2S
At this stage, the amount of sulphur in the naphtha reduces considerably. Now this naphtha is sent to the desulfurization unit. Which has Co & Mo catalyst beds and here the remaining sulphur also gets hydrogenated and converted into H2O. The H2S thus formed is sent to the absorption column. Where there are beds of Zn due to which most of the H2S gets removed.
It is mixed with the purified naphtha obtained from the desulfurisation unit and heated to 500 °C, then the gas is sent to the catalyst tube through the primary remover. The mole ratio of feed steam and carbon is kept at 35%. After the reaction, the product obtained from the primary reformer contains about 10% unconverted CH4, the reaction of which is as follows
C_n H_{2n} + nH ⟶ nCO + (2n+1)H2
CH_4 + H_2O ⟶ CO + 3H_2
CO + H_2O ⟶ CO2 + H2 + Heat
The gas mixture is now sent to the secondary reformer, where air is supplied. From this, the required N2 can be obtained for NH3 production. From the secondary reformer, the gas mixture is sent to the shift converter section. First of all, the gas mixture is passed to the shift converter at high temperatures. The shift converter contains feed and CrO3 as catalysts, which converts CO into CO2, now this synthesis gas contains CO2.
Hence it is taken to the CO2 absorption tower where N2 and H2 are introduced from the bottom of the tower. The absorption solution contains 27% K2CO3, 83% Diethanol amine as activator and 3.2 - 0.5% P2O5 is present in the solution as corrosion resistance. The reaction of K2CO3 is as follows.
K2CO3+ H2O + CO2 ⟶ 2K2CO3
To remove CO2, the absorption solution is stripped, the reaction of which is as follows.
2K2CO3 ⇌ K2CO3+ H2O + CO2
From here, CO2 is obtained and sent to the urea plant. And the absorption solution is sent to the methanator via the absorption tower. Where CO and CO2 are converted into methane. The methanator contains highly active Ni-Co catalysts.
CO2 + 3H2 ⟶ CH4 + H2O + Heat
CO2 + 4H2 ⟶ CH4 + 2H2O+ Heat
After passing the raw synthesis gas from the CO2 absorption tower and methanator, pure synthesis gas is obtained, which is compressed up to 150 kg/cm2 by a synthesis gas compressor and sent to an ammonia (NH3) synthesis unit, in which the pressure is quite high. Now the gas is passed through a water chiller and the liquid ammonia is sent to the urea plant or for storage.
Production of Ammonia (NH3) from Coke Oven Gas
The coal carbonisation plant of coke oven gas steel industries has bi-products, a major plant and machinery for their production - batch type bi-product coke oven gas and gas purifier unit and the basic material is coal blend.