Ammonium Nitrate comes under nitrate fertilizers, it contains about 35% nitrogen. N2 is found in both forms (ammonical & Nitrate) in it. It is used in almost all types of soil and agriculture fields. It is highly hygroscopic.
It contains nitrogen in nitrate form. It is used for direct application, especially for paddy crops. In our country, it is used by converting it into calcium Ammonium nitrate and ammonium sulphate nitrate.
{tocify} $title={Table of Contents}
Properties
Ammonium Nitrate has a tendency of cake formation. Due to this, the product should have less than 1% moisture. Ammonium Nitrate has the property of self-heating and due to this, thermal disintegration occurs at a fast rate. Due to this, there is a fear of fire and explosion.
I. Molecular weight - 80.05
II. Melting point - 170 °C
III. Boiling point - Decompose at 200 °C
IV. Solubility - Soluble in water (900/Ltr)@20°C
V. Percent of Nitrogen - 35%
VI. Specific Gravity - 1.660
VII. Bulk Density - 228.7 kg/m^3
VII. Critical Humidity - 63.3
Raw Materials & Chemical Reaction
i. Nitric acid
ii. Ammonia
iii. Clay.
It is produced by reacting ammonia with nitric acid.
NH3 + HNO3 ⟶ NH4NO3
Method of Production & Process Description
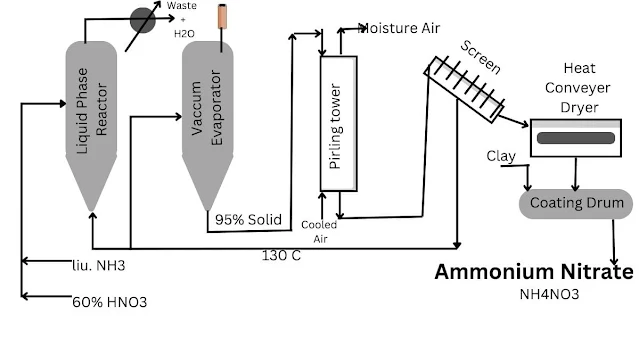
Production of NH4NO3 Solution:- In this, a reactor vessel made of stainless steel is used in which agitators are fitted. This reactor vessel is called a neutralizer. Since the nature of the reaction is exothermic. Hence the heat produced in the reaction is used in steam generation. In which vaporised liquid ammonia is converted into ammonia vapour.
Nitric acid is fed into the neutralizer and at this stage ammonium nitrate solution is formed. The concentration of this solution is about 83%. This solution is continuously fed into a vacuum evaporator at 140°C. The concentration of ammonium nitrate in the vacuum evaporator reaches up to 99%.
Solidification of NH4NO3:- The solution thus obtained is sent to the prilling tower by a pump and it falls down in spray form, and the air is sent upwards by fans installed at the bottom. The height of the prilling tower is about 60 to 75 meters.
The diameter of solidified spherical prills and plates is approximately 15 mm. These prills are screened with the help of a screen. They are oversized and fine particles and are recycled in the neutralizing reactor. This reduces their explosiveness. Then this product is packed in bags.
Storage and Packing
Ammonium Nitrate prills are freely dispersed. Therefore, generally, there is no problem in its storage and handling. But it should be handled with great care because when it mixes with any combustible substance or gets heated to a high temperature, it can explode.
Operational Problem in Plant
(i). Only carbon steel is used for NH3 storage and feed system. For 120°C equivalent or 100% HNO3, extra low-carbon steel can be used.
(ii). Due to the high activity of Ammonium Nitrate, safety precautions are necessary, such as drying it, air free from oil and flammable substances is required.
(iii). Ammonium Nitrate is an explosive component. Hence equipment is designed in which residence should be for a short time.