Apart from the mechanical properties of materials stated so far, another important property is corrosion resistance. All metals are thermodynamically unstable and tend to react with their environment to produce compounds such as oxides or carbonates.
This reaction involves the movement of electrons and is called an electrochemical reaction.
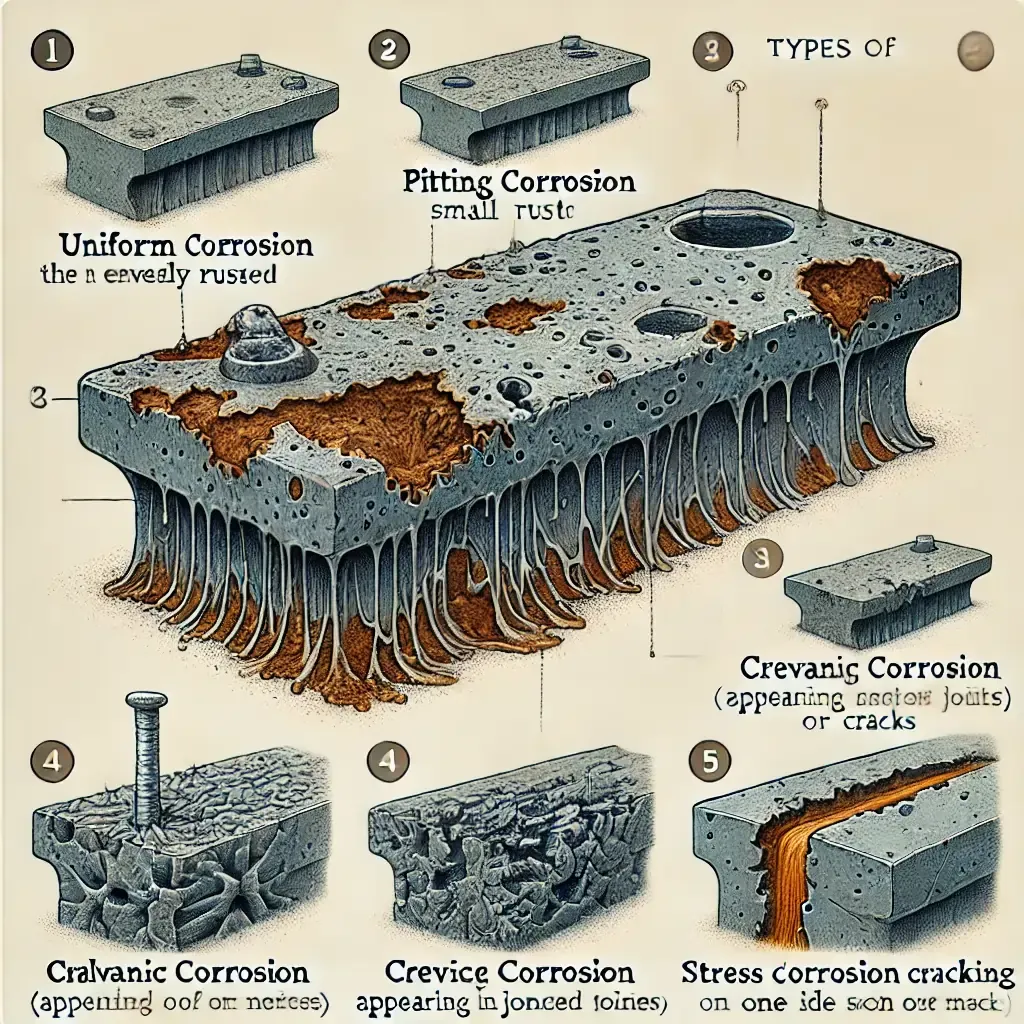
Types of Corrosions
Uniform Corrosion
This is the
most common form of corrosion, characterized by the chemical or electrochemical reaction that proceeds evenly and uniformly over the entire
exposed area. It is indicated by the general wasting of the surface. The corrosive
product may either form a protective layer on the metal or, in the case of a direct chemical attack, the corroded material will dissolve in the corrosive environment.
This type of corrosion can be prevented or reduced by (i) Coating, (ii)
Inhibitors, or (iii) Cathodic protection.
Galvanic corrosion
This occurs when two dissimilar metals are placed in contact. The less resistant metal becomes anodic, and the more resistant metal becomes cathodic. Usually, the corrosion of cathodic metal is nil or very little in this type of couple. For example, in heat exchangers with copper tubes and cast iron or steel tube sheets, if galvanic corrosion occurs, it accelerates the attack on heavy tube sheets instead of thin copper tubes.
- Select the combination of metals as close together as possible in the galvanic series.
- Avoid the unfavourable area effect of a small anode and a large cathode.
- Insulate dissimilar metals whenever possible.
- Apply coating with caution.
- Add inhibitors, if possible, to decrease the aggressiveness of the environment.
- Avoid threaded joints for materials far apart in the galvanic series.
- Design a system for ease of replacement of anodic material. Also, one can have a thicker anode.
- Provide for cathodic protection.
Crevice
Corrosion
This is
intense localized corrosion that occurs within crevices and other shielded
areas on metal surfaces exposed to corrosives. This type of attack is usually
associated with small volumes of stagnant solution caused by holes, gasket
surfaces, lap joints, surface deposits and crevices under bolt and rivet heads.
Stainless steels are particularly susceptible to revice attack.
Pitting Corrosion
This occurs
between surfaces that are in close contact. It is extremely localized and occurs
due to the presence of impurities, rough spots and scratches. Pitting seldom occurs
with extreme suddenness. It occurs due to stagnant conditions, which implies
that in equipment where a conditions flow of liquid is present, it often exhibits
reduced pitting. Adding inhibitors to metals helps reduce pitting.
Intergranular corrosion
This consists
of a localized attack or an intercrystallite cracking along the grain
boundaries of the metal. It is caused by impurities at the grain boundaries, enrichment of one of the alloying elements or a depletion of one of these
elements in the grain-boundary areas.
Selective leaching
In this
type of corrosion, one element of a metal or alloy is singled out for attack. The
common types are dezincification, dealuminations and graphite erosion. For
example, if copper zinc alloys containing less than 85% copper are exposed to
wet conditions, this type of corrosion occurs for redeposited copper that has little
mechanical strength.
Stress corrosion
This
corrosion is the result of internal or external stresses and a corrosive
environment. It manifests itself in the form of cracks, and it is therefore often
known as stress corrosion cracking. Many metals and some plastics suffer from
this effect. With plastics, it is called environmental cracking. The important
variables affecting stress corrosion cracking are temperature, solution
composition, metal composition structure and stress.
Fatigue Corrosion
As in the
case of stress corrosion, where static stresses are linked up corrosion in this
type of corrosion cyclic load combine with corrosion to cause corrosion
fatigue. Usually, fatigue failures occur at stress levels belowthe yield point and
after many cyclic applications of this stress.
Erosion Corrosion
This is caused
by the combination of corrosive fluid and mechanical wear resulting from the impingement
of liquid or abrasion of solid particles. This effect is mainly dependent on
the liquid velocity and on account of the contained air solids or any factors
that affect the rate of formation of protective films. This process removes layers
from the surface of the metal as dissolved ions form from the metal surface.
Gravitation Corrosion
This is a
special form of corrosion that occurs due to the repeated collapse of vapour
bubbles on a metal surface. This causes mild physical damage to the protective films and severe deformation and fracture of the surface. Cavitation occurs in equipment
where a high-velocity liquid flows and pressure changes are encountered, such as
pump impellers, resulting in vapour bubbles.
Fretting Corrosion
This occurs
at the contact areas between materials under load subjected to vibration and
slip. It appears as pits or grooves in the metal surrounded by corrosion
products. Fretting is also called friction oxidation or wear oxidation.
This type
of corrosion can be minimized by lubricating contact areas or by increasing
the hardness of one or both of the contacting materials.
Hydrogen Damage
This refers to the mechanical damage of metal caused by the presence of or by the interaction with hydrogen. This can be in the form of hydrogen embrittlement decarburization or hydrogen attack.